How Do You Know if Something Is More Soluble in Water or Organic
1.vi: Concrete properties of organic compounds
- Page ID
- 170407
An understanding of the diverse types of noncovalent forces allows the states to explain, on a molecular level, many observable physical properties of organic compounds. In this section, we will concentrate on solubility (peculiarly solubility in water), melting point, and boiling point.
Solubility
Virtually all of the organic chemistry that you will see in this grade takes place in the solution phase. In the organic laboratory, reactions are often run in nonpolar or slightly polar solvents such every bit toluene (methylbenzene), dichloromethane, or diethylether. In recent years, much effort has been made to adapt reaction conditions to permit for the apply of 'greener' (in other words, more environmentally friendly) solvents such equally water or ethanol, which are polar and capable of hydrogen bonding. In biochemical reactions the solvent is of class water, but the 'microenvironment' inside an enzyme's active site - where the actual chemistry is going on - tin range from very polar to very not-polar, depending on which amino acid residues are nowadays.
You probably remember the 'similar dissolves like' dominion y'all learned in general chemistry, and fifty-fifty earlier you took any chemistry at all, you probably observed at some point in your life that oil does not mix with water. Allow'southward revisit this rule, and put our cognition of covalent and noncovalent bonding to piece of work.
When considering the solubility of an organic compound in a given solvent, the most important question to ask ourselves is: how strong are the noncovalent interactions between the chemical compound and the solvent molecules? If the solvent is polar, similar water, then a smaller hydrocarbon component and/or more charged, hydrogen bonding, and other polar groups volition tend to increase the solubility. If the solvent is not-polar, similar hexane, and then the exact contrary is true.
Imagine that y'all have a flask filled with h2o, and a selection of substances that you will test to come across how well they dissolve in the water. The beginning substance is tabular array common salt, or sodium chloride. As y'all would virtually certainly predict, especially if you lot've ever inadvertently taken a mouthful of water while swimming in the sea, this ionic chemical compound dissolves readily in h2o. Why? Because water, as a very polar molecule, is able to course many ion-dipole interactions with both the sodium cation and the chloride anion, the energy from which is more than enough to make up for free energy required to break up the ion-ion interactions in the salt crystal.
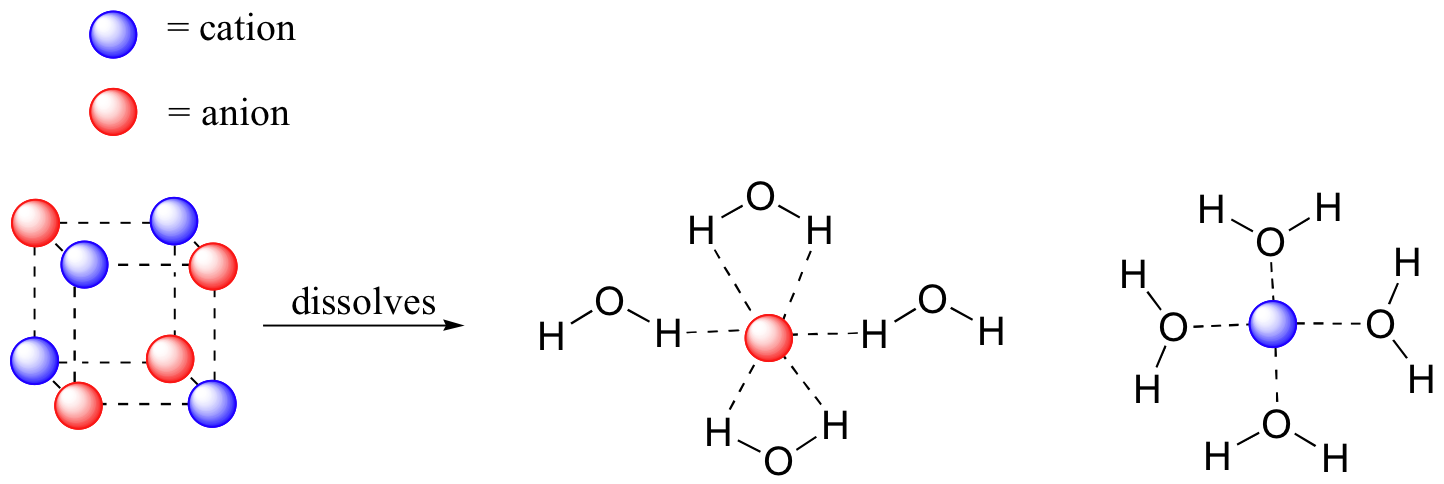
The end event, then, is that in identify of sodium chloride crystals, nosotros take private sodium cations and chloride anions surrounded by water molecules – the salt is now in solution. Charged species as a rule dissolve readily in h2o: in other words, they are very hydrophilic (water-loving).
Now, we'll try a compound called biphenyl, which, like sodium chloride, is a colorless crystalline substance.
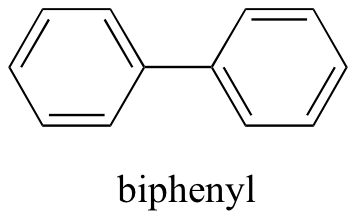
Biphenyl does not dissolve at all in water. Why is this? Because it is a very not-polar molecule, with just carbon-carbon and carbon-hydrogen bonds. It is able to bond to itself very well through nonpolar van der Waals interactions, simply information technology is non able to class pregnant bonny interactions with very polar solvent molecules similar h2o. Thus, the energetic price of breaking up the biphenyl-to-biphenyl interactions in the solid is loftier, and very little is gained in terms of new biphenyl-water interactions. Water is a terrible solvent for nonpolar hydrocarbon molecules: they are very hydrophobic (h2o-fearing).
Side by side, you try a serial of increasingly big alcohol compounds, starting with methanol (one carbon) and ending with octanol (8 carbons).
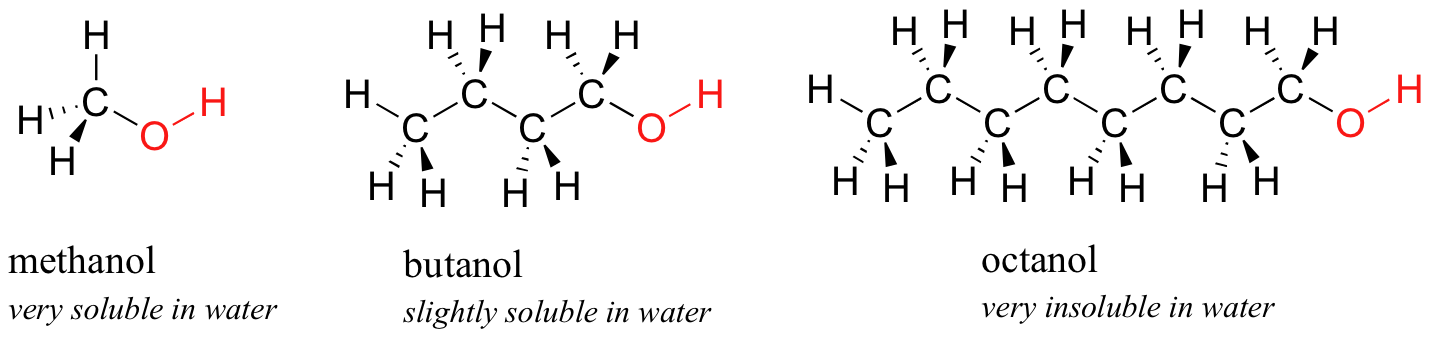
You lot observe that the smaller alcohols - methanol, ethanol, and propanol - dissolve easily in h2o, at whatsoever water/alcohol ratio that you try. This is because the water is able to course hydrogen bonds with the hydroxyl group in these molecules, and the combined energy of formation of these water-booze hydrogen bonds is more than enough to make up for the energy that is lost when the alcohol-alcohol (and water-water) hydrogen bonds are broken upwards. When you attempt butanol, all the same, you begin to detect that, as you add more and more than to the water, it starts to grade a layer on top of the water. Butanol is just sparingly soluble in water.
The longer-chain alcohols - pentanol, hexanol, heptanol, and octanol - are increasingly non-soluble in water. What is happening here? Clearly, the same favorable water-alcohol hydrogen bonds are still possible with these larger alcohols. The divergence, of course, is that the larger alcohols have larger nonpolar, hydrophobic regions in addition to their hydrophilic hydroxyl group. At most four or five carbons, the influence of the hydrophobic part of the molecule begins to overcome that of the hydrophilic part, and h2o solubility is lost.
Now, endeavour dissolving glucose in the water – even though it has six carbons just similar hexanol, it also has 5 hydrogen-bonding, hydrophilic hydroxyl groups in addition to a sixth oxygen that is capable of being a hydrogen bail acceptor.
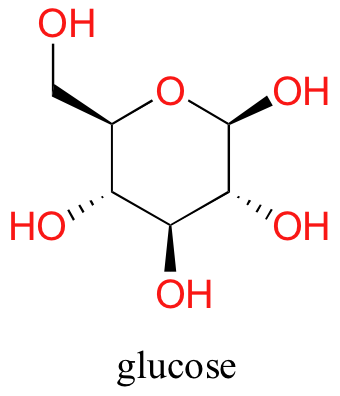
Nosotros accept tipped the scales to the hydrophilic side, and we notice that glucose is quite soluble in water.
We saw that ethanol was very water-soluble (if it were not, drinking beer or vodka would be rather inconvenient!) How about dimethyl ether, which is a constitutional isomer of ethanol but with an ether rather than an booze functional group? We detect that diethyl ether is much less soluble in water. Is information technology capable of forming hydrogen bonds with water? Yeah, in fact, it is –the ether oxygen tin can deed every bit a hydrogen-bail acceptor. The difference betwixt the ether group and the alcohol group, nonetheless, is that the alcohol group is both a hydrogen bond donor and acceptor.
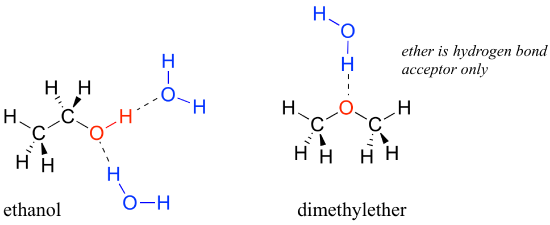
The consequence is that the alcohol is able to form more energetically favorable interactions with the solvent compared to the ether, and the alcohol is therefore much more than soluble.
Here is another easy experiment that tin be done (with proper supervision) in an organic laboratory. Attempt dissolving benzoic acid crystals in room temperature water – you lot'll find that it is not soluble. As we volition learn when we report acid-base of operations chemistry in a later chapter, carboxylic acids such as benzoic acrid are relatively weak acids, and thus be mostly in the acidic (protonated) form when added to pure h2o.
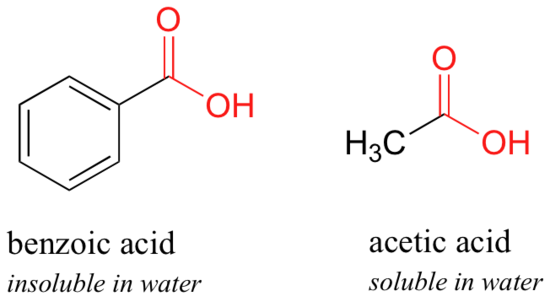
Acerb acid (vinegar) is quite soluble. This is piece of cake to explicate using the small alcohol vs large alcohol argument: the hydrogen-bonding, hydrophilic effect of the carboxylic acrid group is powerful enough to overcome the hydrophobic outcome of a unmarried hydrophobic methyl grouping on acetic acid, merely not the larger hydrophobic effect of the 6-carbon benzene grouping on benzoic acrid.
Now, try slowly adding some aqueous sodium hydroxide to the flask containing undissolved benzoic acid. As the solvent becomes more and more bones, the benzoic acrid begins to dissolve, until it is completely in solution.
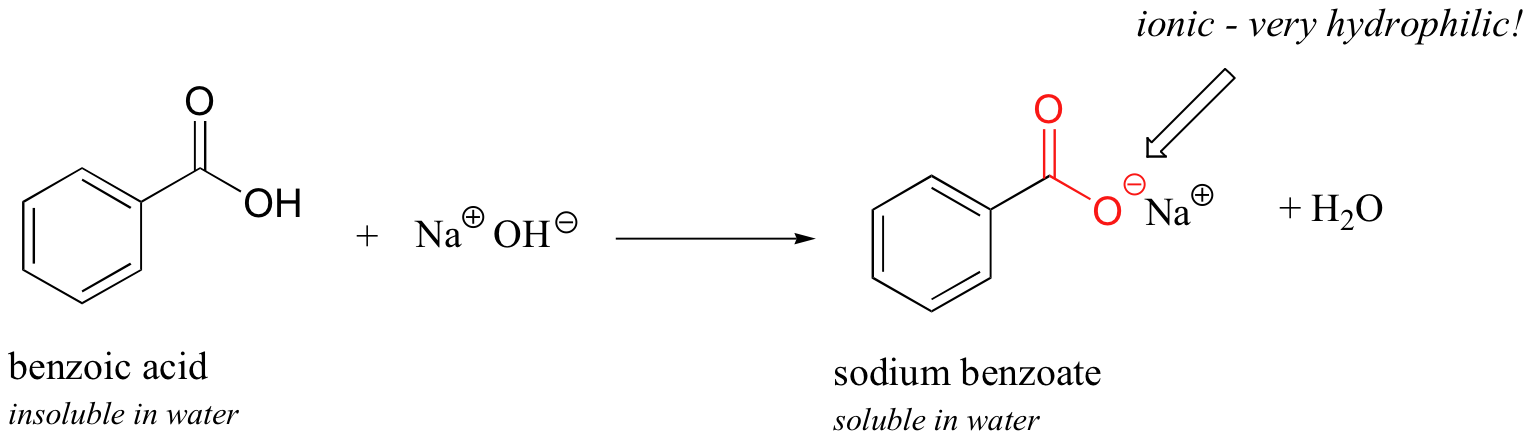
What is happening here is that the benzoic acid is beingness converted to its conjugate base of operations, benzoate. The neutral carboxylic acid group was not hydrophilic enough to make up for the hydrophobic benzene ring, but the carboxylate group, with its full negative charge, is much more hydrophilic. At present, the balance is tipped in favor of water solubility, every bit the powerfully hydrophilic anion function of the molecule drags the hydrophobic function into solution. Remember, charged species usually dissolve readily in water. If y'all want to precipitate the benzoic acid back out of solution, you can simply add enough hydrochloric acid to neutralize the solution and reprotonate the carboxylate.
If you are taking a lab component of your organic chemistry form, you will probably do at least one experiment in which y'all will use this phenomenon to physically separate an organic acid like benzoic acid from a hydrocarbon chemical compound like biphenyl.
Similar arguments can be made to rationalize the solubility of different organic compounds in nonpolar or slightly polar solvents. In general, the greater the content of charged and polar groups in a molecule, the less soluble it tends to be in solvents such equally hexane. The ionic and very hydrophilic sodium chloride, for example, is not at all soluble in hexane solvent, while the hydrophobic biphenyl is very soluble in hexane.
Because we are concentrating on the biologically relevant chemistry, let's have a minute to review how to evaluate a compound's solubility in water, the biological solvent:
A: How many carbons? All else being equal, more carbons means more of a non-polar/hydrophobic character, and thus lower solubility in h2o.
B: How many, and what kind of hydrophilic groups? The more, the greater the water solubility. In social club of importance:
- Annihilation with a charged group (eg. ammonium, carboxylate, phosphate) is almost certainly water soluble, unless has a vary big nonpolar grouping, in which instance it volition near likely be soluble in the grade of micelles, like a soap or detergent (see side by side section).
- Whatsoever functional group that tin can donate a hydrogen bond to h2o (eg. alcohols, amines) will significantly contribute to water solubility.
- Whatsoever functional group that can only accept a hydrogen bail from water (eg. ketones, aldehydes, ethers) will take a somewhat smaller but still meaning effect on water solubility.
- Other groups that contribute to polarity (eg. alkyl halides, thiols sulfides) will make a pocket-size contribution to water solubility.
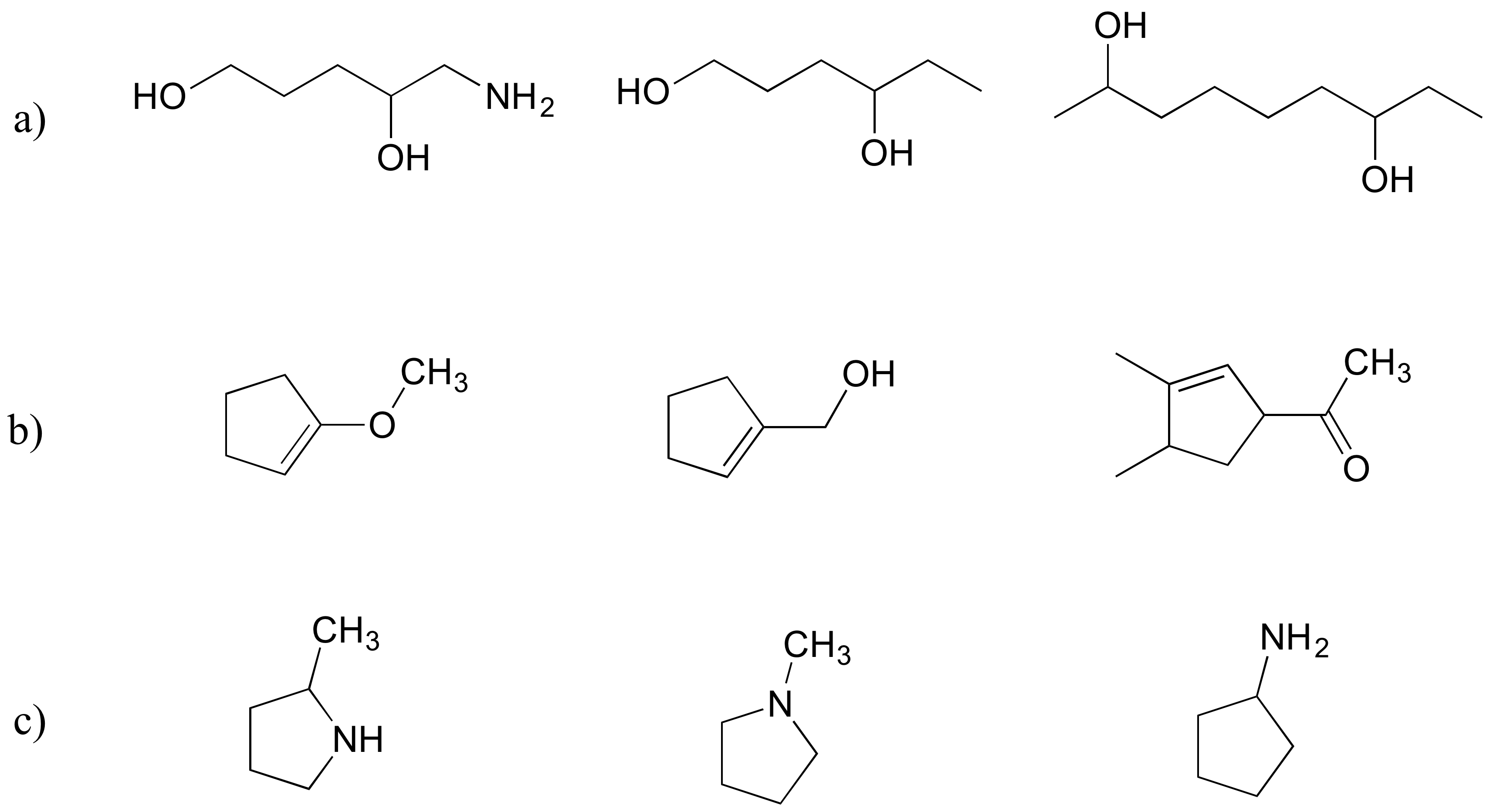
Exercise 2.30
Rank each set of iii compounds below according to their solubility in water (most soluble to to the lowest degree):
Exercise 2.31
Vitamins tin can be classified every bit water-soluble or fat-soluble (consider fat to be a very non-polar 'solvent'. Make up one's mind on a classification for each of the vitamins shown beneath.
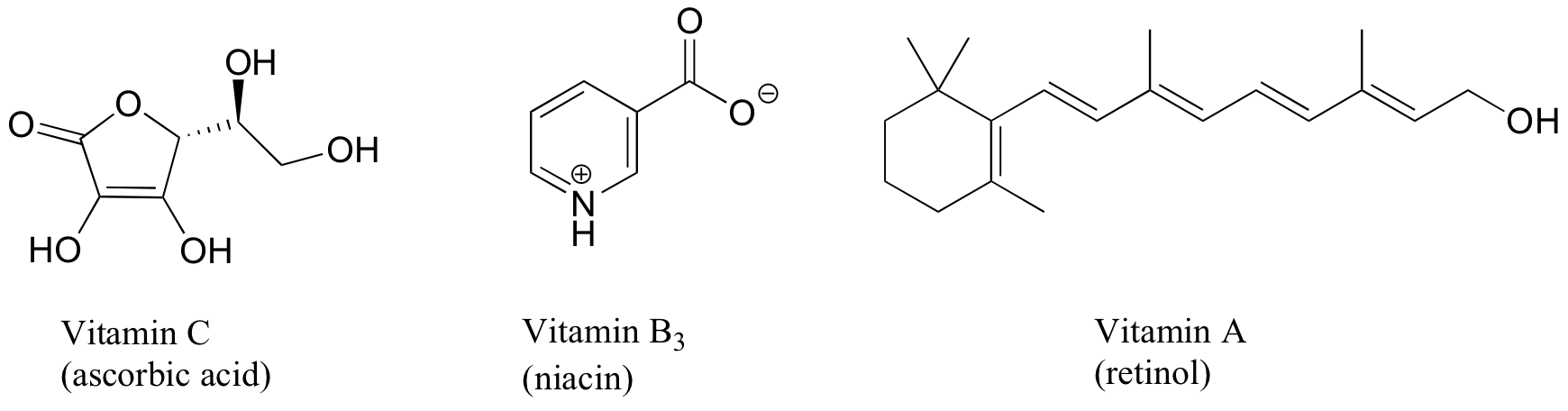
Exercise 2.32
Both aniline and phenol are more often than not insoluble in pure h2o. Predict the solubility of these two compounds in 10% aqueous muriatic acid, and explicate your reasoning.

Exercise 2.33
Would you lot predict methanol or 2-propanol (rubbing booze) to exist a better solvent for cyclohexanone? Why?
Solutions to exercises
Because h2o is the biological solvent, about biological organic molecules, in gild to maintain h2o-solubility, contain ane or more charged functional groups: nearly often phosphate, ammonium or carboxylate.
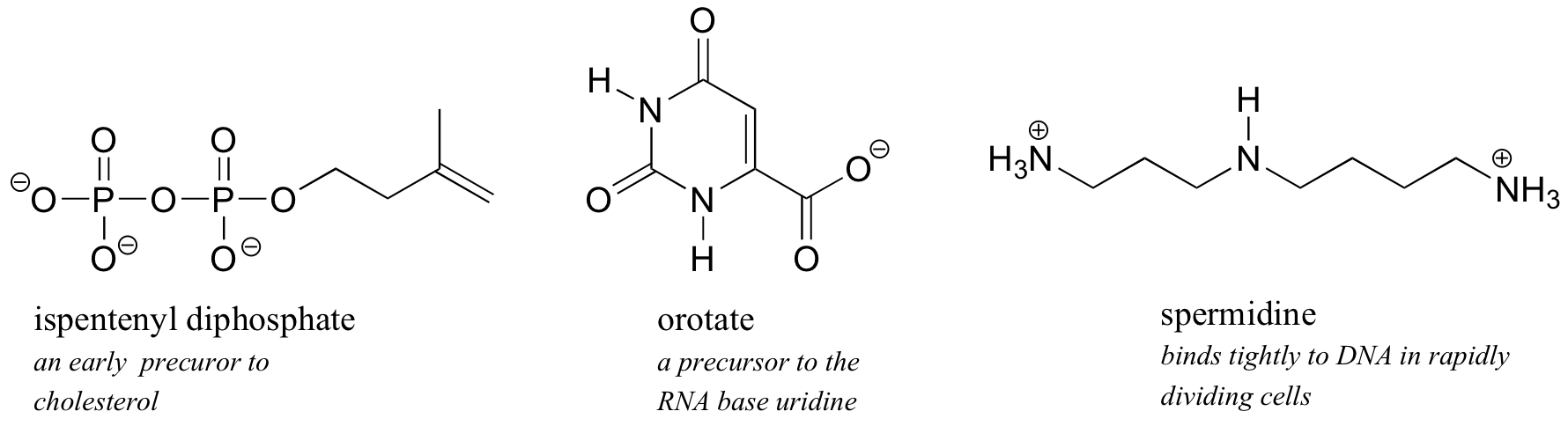
Note that the accuse on these functional groups depends on their protonation state: spermidine, for example, could be drawn with 3 (uncharged) amine groups rather than the charged ammonium groups as shown, and orotate could exist drawn in the uncharged carboxylic acid form. It turns out, however, that these three functional groups are all charged when in a buffer at the physiological pH of approximately 7.iii. We will take much more to say nearly the acrid-base aspects of these groups in affiliate seven.
Carbohydrates often lack charged groups, but as we discussed in our 'idea experiment' with glucose, they are quite water-soluble due to the presence of multiple hydroxyl groups, which can hydrogen bond with water.
Some biomolecules, in contrast, incorporate distinctly hydrophobic components. Membrane lipids are amphipathic, meaning that they contain both hydrophobic and hydrophilic components. Cell membranes are composed of membrane lipids arranged in a 'bilayer', with the hydrophobic 'tails' pointing in and the hydrophilic 'heads' forming the inner and outer surfaces, both of which are in contact with h2o.
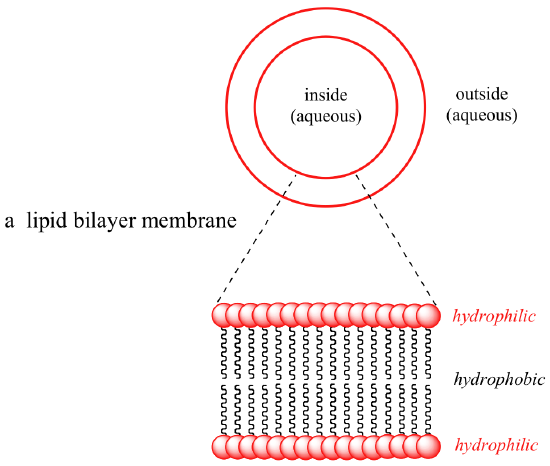
Interactive 3D images of a fatty acrid lather molecule and a soap micelle (Edutopics)
The nonpolar interior of the lipid bilayer is able to 'dissolve' hydrophobic biomolecules such as cholesterol. Polar and charged biomolecules, on the other hand, are not able to cantankerous the membrane, because they are repelled past the hydrophobic environment of the bilayer'due south interior. The transport of water-soluble molecules beyond a membrane tin exist achieved in a controlled and specific mode by special transmembrane ship proteins, a fascinating topic that y'all volition learn more well-nigh if you take a class in biochemistry.
A similar principle is the basis for the action of soaps and detergents. Soaps are equanimous of fatty acids such every bit stearate obtained through basic hydrolysis of triacylglycerols in fats and oils.
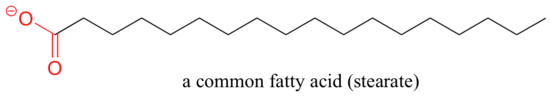
Like membrane lipids, fatty acids are amphipathic. In aqueous solution, the fat acrid molecules in soaps will spontaneously grade micelles, a spherical structure that allows the hydrophobic tails to avoid contact with h2o and simultaneously form favorable van der Waals contacts with each other.
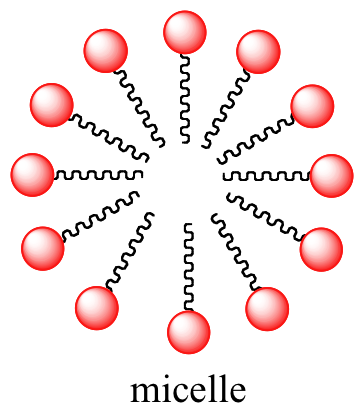
Interactive 3D images of a fatty acid soap molecule and a soap micelle (Edutopics)
Because the outside of the micelle is charged, the structure as a whole is soluble in water. Micelles will form spontaneously around pocket-size particles of oil that normally would non dissolve in water, and will carry the particle away with it into solution. We will acquire more most the chemistry of soap-making in chapter 11.
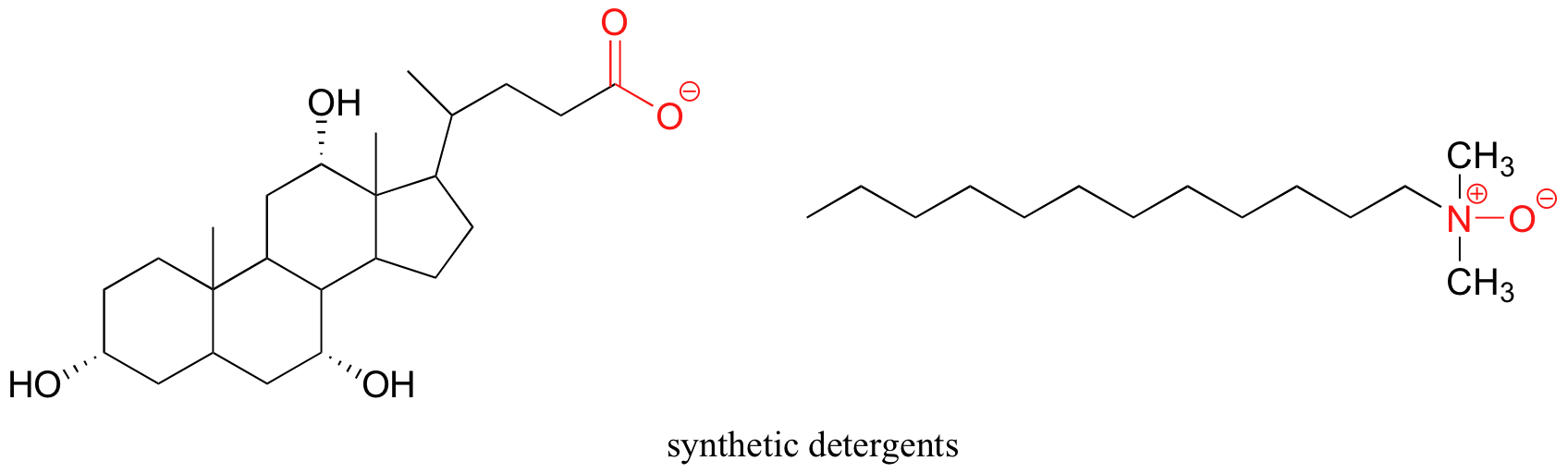
Synthetic detergents are non-natural amphipathic molecules that piece of work by the same principle as that described for soaps.
Humid point and melting point
The appreciable melting and boiling points of dissimilar organic molecules provides an boosted analogy of the furnishings of noncovalent interactions. The overarching principle involved is elementary: how well can a chemical compound bind to itself? Melting and humid are processes in which noncovalent interactions between identical molecules in a pure sample are disrupted. The stronger the noncovalent interactions, the more than energy that is required, in the form of heat, to interruption them apart.
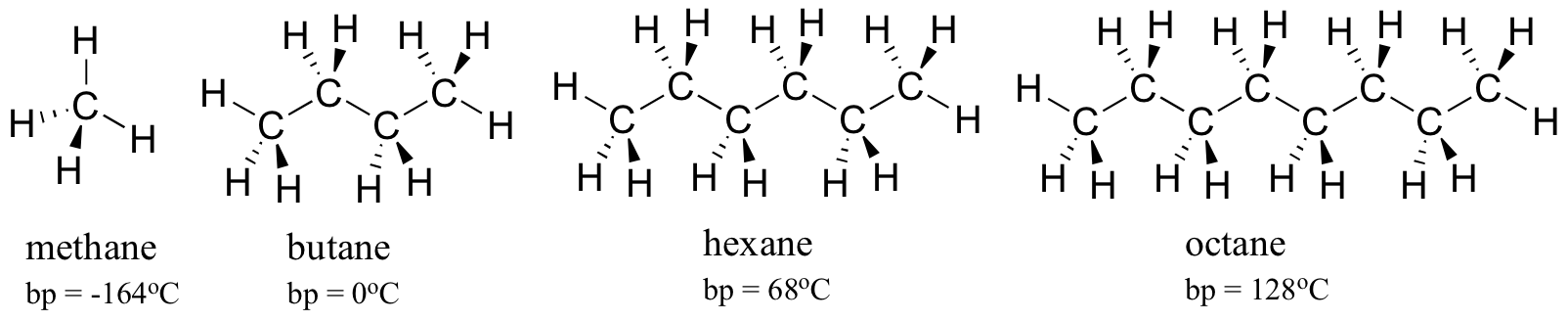
Every bit a rule, larger molecules take higher humid (and melting) points. Consider the boiling points of increasingly larger hydrocarbons. More carbons and hydrogens means a greater expanse possible for van der Waals interaction, and thus higher boiling points. Beneath zero degrees centigrade (and at atmospheric pressure level) butane is a liquid, because the butane molecules are held together by Van der Waals forces. Above zero degrees, however, the molecules proceeds plenty thermal energy to intermission autonomously and enter the gas phase. Octane, in contrast, remains in the liquid phase all the way up to 128oC, due to the increased van der Waals interactions fabricated possible by the larger surface area of the individual molecules.
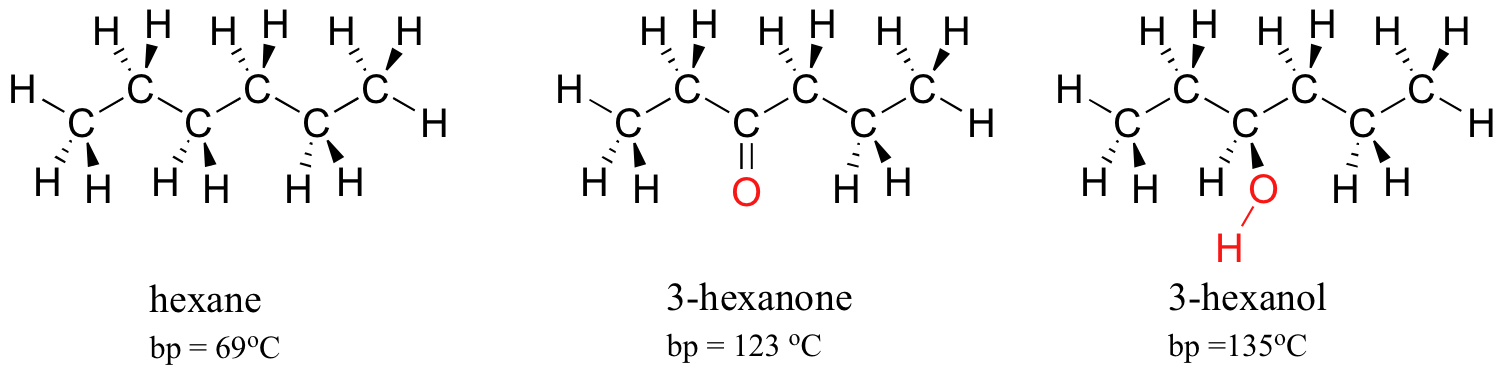
The force of intermolecular hydrogen bonding and dipole-dipole interactions is reflected in higher boiling points. Look at the trend for hexane (van der Waals interactions only), 3-hexanone (dipole-dipole interactions), and three-hexanol (hydrogen bonding). In all three molecules, van der Waals interactions are meaning. The polar ketone group allows 3-hexanone to form intermolecular dipole-dipole interactions, in addition to the weaker van der Waals interactions. three-hexanol, because of its hydroxyl group, is able to form intermolecular hydrogen bonds, which are stronger yet.
Of particular involvement to biologists (and pretty much anything else that is alive on the planet) is the upshot of hydrogen bonding in water. Considering it is able to form tight networks of intermolecular hydrogen bonds, water remains in the liquid phase at temperatures up to 100 OC despite its small size. The world would evidently exist a very unlike identify if h2o boiled at xxx OC.
Based on their structures, rank phenol, benzene, benzaldehyde, and benzoic acid in terms of lowest to highest humid signal. Explain your reasoning.
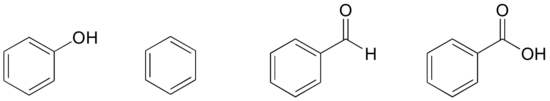
Solutions to exercises
By thinking about noncovalent intermolecular interactions, nosotros tin likewise predict relative melting points. All of the aforementioned principles apply: stronger intermolecular interactions result in a higher melting point. Ionic compounds, as expected, usually have very loftier melting points due to the strength of ion-ion interactions. Just similar with boiling points, the presence of polar and hydrogen-bonding groups on organic compounds by and large leads to higher melting points. The size of a molecule influences its melting point too equally its humid point, over again due to increased van der Waals interactions betwixt molecules.
What is different about melting point trends, that we don't see with humid point or solubility trends, is the importance of a molecule's shape and its power of pack tightly together. Moving-picture show yourself trying to make a stable pile of baseballs in the flooring. It merely doesn't work, because spheres don't pack together well - at that place is very picayune area of contact between each ball. It is very like shooting fish in a barrel, though, to brand a stack of flat objects similar books.
The aforementioned concept applies to how well molecules pack together in a solid. The flat shape of aromatic compounds allows them to pack efficiently, and thus aromatics tend to have higher melting points compared to non-planar hydrocarbons with like molecular weights. Comparing the melting points of benzene and toluene, you lot can see that the extra methyl group on toluene disrupts the molecule'due south ability to pack tightly, thus decreasing the cumulative strength of intermolecular van der Waals forces and lowering the melting signal.
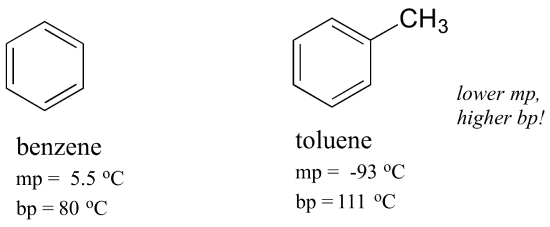
Annotation also that the boiling signal for toluene is significantly higher up the boiling signal of benzene! The key factor for the humid point trend in this case is size (toluene has ane more carbon), whereas for the melting point tendency, shape plays a much more than important office. This makes sense when you consider that melting involves 'unpacking' the molecules from their ordered assortment, whereas boiling involves only separating them from their already loose (liquid) association with each other.
Concrete backdrop of lipids and proteins
Lipids
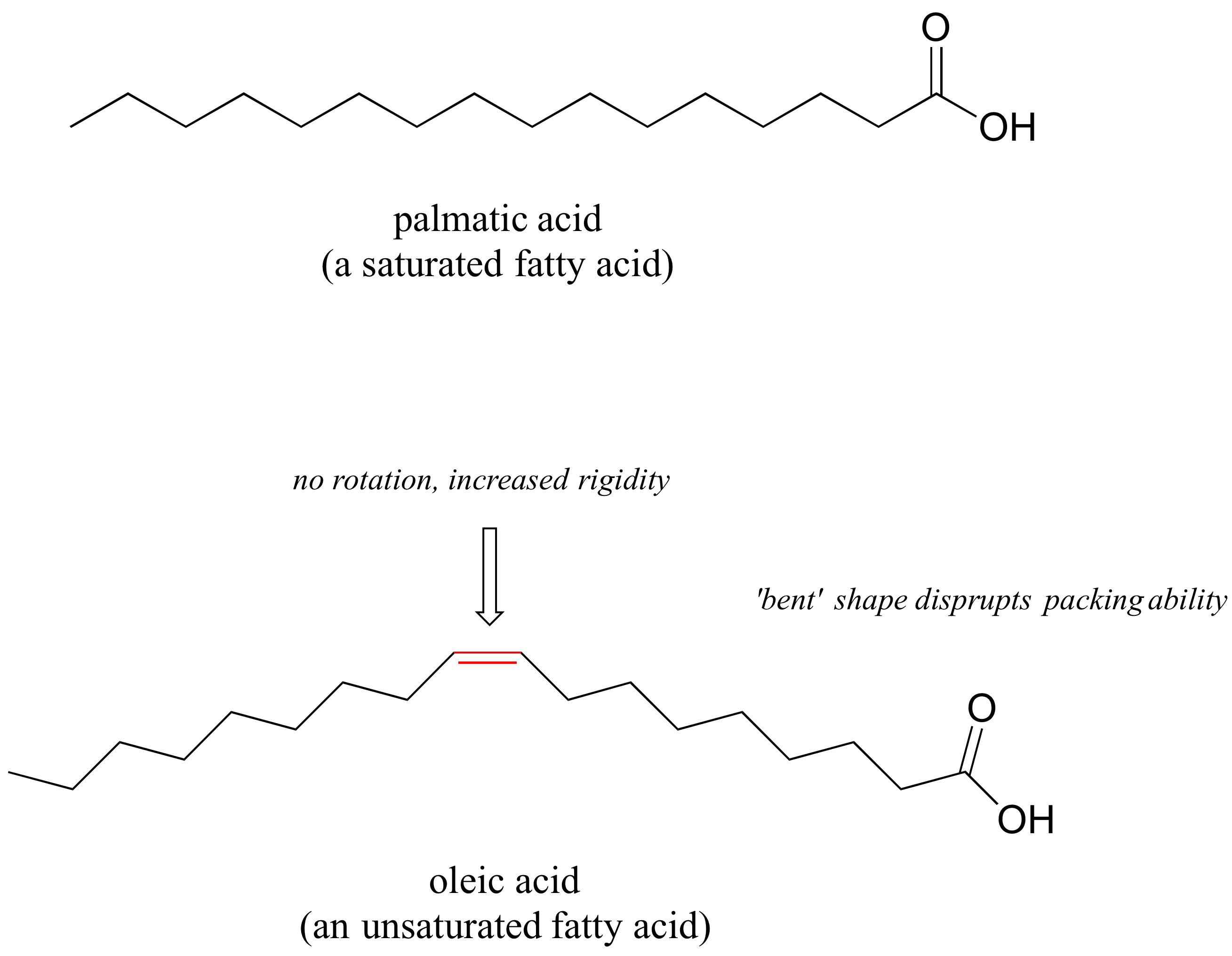
An interesting biological case of the relationship between molecular structure and melting betoken is provided past the observable physical deviation between animal fats like butter or lard, which are solid at room temperature, and vegetable oils, which are liquid. Call up that fats and oils are triacylglycerols: fat acids linked to a glycerol backbone. In vegetable oils, the fatty acrid components are unsaturated, pregnant that they contain one or more than double bonds. Solid creature fatty, in contrast, contains mainly saturated hydrocarbon bondage, with no double bonds.
Interactive 3D image of a saturated triacylglycerol (BioTopics)
Saturated vs mono-unsaturated fatty acid (BioTopics)
The double bond(due south) in vegetable oils cause those hydrocarbon bondage to be more rigid, and 'aptitude' at an angle (remember that rotation is restricted around double bonds), with the consequence that they don't pack together equally closely, and thus can exist broken apart (melted) more readily.
In a related context, the fluidity of a cell membrane (substantially, the melting point) is determined to a large extent by the length and degree of unsaturation of the fatty acrid 'tails' on the membrane lipids. Longer and more saturated fatty acids make the membrane less fluid (they are able maximize van der Waals interactions), while shorter and more unsaturated fatty acids cause the membrane to be more fluid.
Proteins
The very same noncovalent forces we have simply learned well-nigh are also integral to protein structure: when a protein folds up, it does and so in such a mode that very specific non-covalent interactions form between amino acid residues on different regions of the chain, each one becoming office of the 'molecular glue' that holds the concatenation together in its correctly folded shape. Hydrogen bonds and charge-accuse interactions are specially important in this respect. In general, the interior of a folded protein is relatively hydrophobic, while the outside surface, which of class is in constant contact with water, is very hydrophilic - many charged side chains such as aspartate, glutamate, lysine, and arginine signal out of the surface of a protein structure.
Most of the proteins of 'mesophilic' organisms (those who thrive in intermediate temperatures, including humans) will denature - come up unfolded - at high temperatures, equally the heat disrupts the specific noncovalent interactions property the protein chain together. Unfolded proteins ordinarily are non water soluble because the more than hydrophobic interior regions are no longer hidden from the solvent, and then denaturing is accompanied by atmospheric precipitation. Obviously, an unfolded protein as well loses its functionality.
In the last few decades, we have become aware that a wide variety of microbes naturally inhabit extremely hot environments such every bit the humid water of hot springs in Yellowstone National Park, or the base of a abyssal thermal vent. How exercise the proteins of these 'thermophiles' agree up to the estrus? There is nothing extraordinary near these proteins that makes them then resistant to oestrus, other than the fact that they accept evolved so that they simply have more molecular 'gum' belongings them together - in particular, more ionic interactions between oppositely charged residues. In simply one of many examples, the iii-dimensional structure of an enzyme from Pyrococcus horikoshii, a microbe isolated from a thermal vent deep in the Pacific Body of water, was compared to a very similar enzyme in humans. The thermophilic protein has a stabilizing charge-accuse interaction between the final carboxylate group on the last amino acid in the chain and an arginine residue near the beginning of the chain.
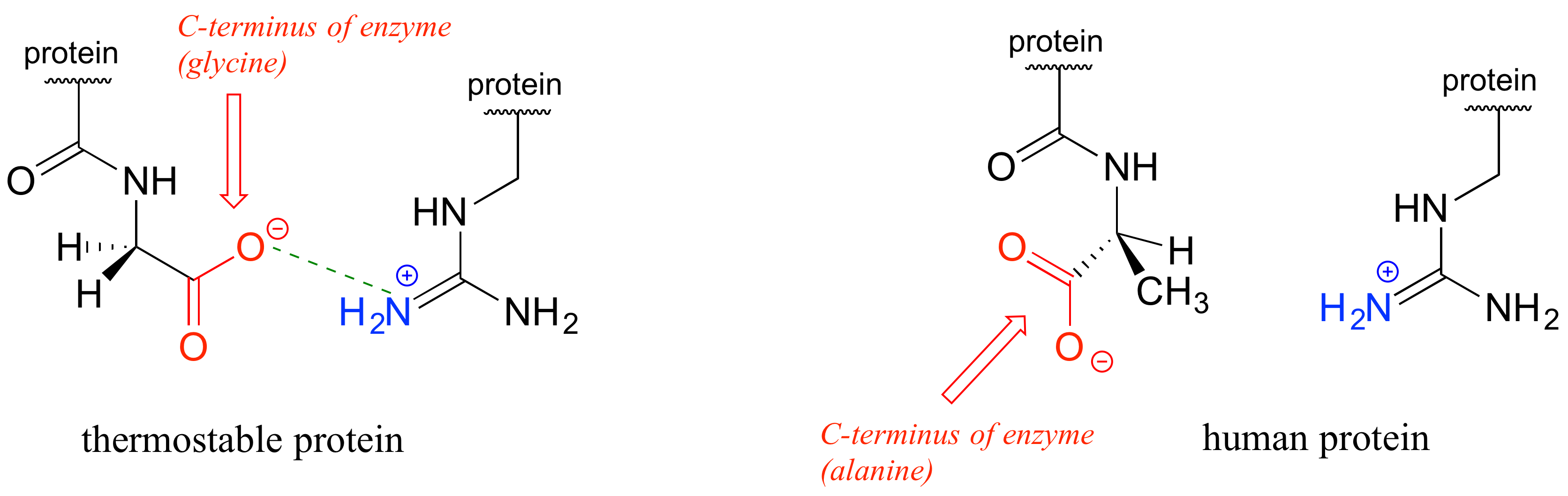
This interaction is not present in the human being version of the protein because the final carboxylate group is angled away from the positively-charged group on the arginine. The single charge-charge interaction is not past itself responsible for the thermostability of the P. horikoshii protein - other similar interactions throughout the poly peptide structure also contribute (encounter the original report at PLOS Biology 2011, 9, e1001027).
Conversely, proteins from 'psychrophilic' organisms - those which alive in extremely cold temperatures, such equally in arctic soils or in minor h2o pockets in polar ice - accept fewer stabilizing charge-accuse interactions. This gives them the flexibility to function at temperatures in which mesophilic human or E. coli proteins would be frozen and inactive. On the other hand, a typical psychrophilic protein will rapidly unfold, precipitate, and lose its functionality at room temperature.
Scientists are extremely interested in thermostable proteins, because the ability to role at loftier temperatures can be a very desirable trait for a protein used in industrial processes. In fact, thermostable Deoxyribonucleic acid polymerase from Thermus aquaticus (the enzyme is known to molecular biologists as 'Taq polymerase') is the enzyme that makes the PCR (polymerase chain reaction) process possible, and has earned billions of dollars in royalties for drug visitor Hoffman La Roche, the patent owner. Many research groups are searching for useful enzymes in thermophilic species, and others are working on ways to engineer heat stability into existing mesophilic enzymes past tinkering with their amino acid sequences to introduce new stabilizing charge-accuse interactions.
Khan Academy video tutorials on solubility, boiling point
robbinsthornested.blogspot.com
Source: https://chem.libretexts.org/Courses/Westminster_College/CHE_261_-_Organic_Chemistry_I/01:_Organic_Structures_and_Bonding/1.6:_Physical_properties_of_organic_compounds

Post a Comment for "How Do You Know if Something Is More Soluble in Water or Organic"